Abstract
Introduction: Plasma cell leukemia (PCL) is an aggressive rare leukemic variant of multiple myeloma (MM). PCL without prior evidence of MM is termed primary PCL (PPCL) and PCL evolving from a pre-existing MM is termed secondary PCL (SPCL). Prognosis for PCL has improved with Immunomodulatory drugs (IMDs) and Bortezomib based chemotherapy, followed by Autologous Stem Cell Transplantation (ASCT). We are presenting 4 years data on clinical profile and treatment outcomes of primary PCL patients from our cancer center from Northern India.
Material and Methods: We retrospectively reviewed medical records of 240 multiple myeloma patients at our center from year 2013 to 2016 year, out of which 9 patients were diagnosed with primary PCL.
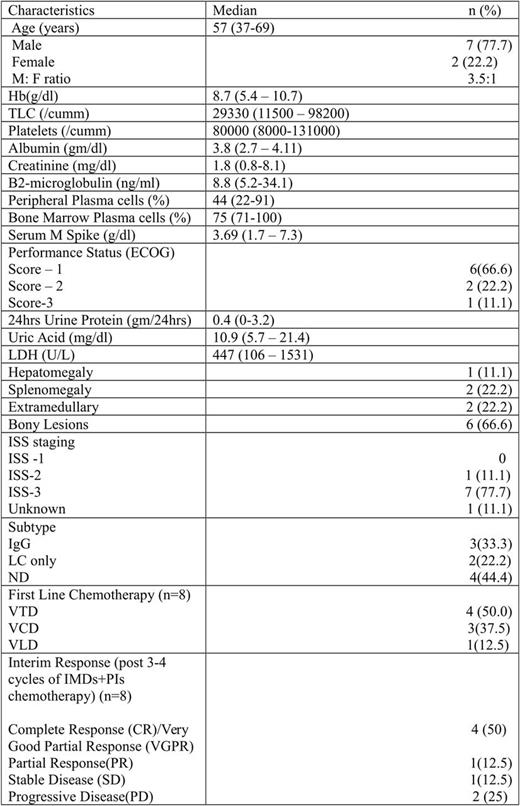
Results: Total 9 patients (7 males) were diagnosed with primary PCL during study period, accounting for 3.75% of all myeloma patients. Median age was 57 yrs (37-69yrs) and median duration of symptoms was 3 months (1-8 months) at the time of diagnosis. Most common presenting complaints were bodyache and bony pains followed by weakness, weight loss and fever. Anemia was present in all patients; renal insufficiency, hypercalcemia and bone lesions were present in 4, 3 and 6 patients respectively. Extramedullary disease was present in 2 patients, hyperuricemia in 7, raised LDH in 7 patients. Seven patients were ISS stage III (Table 1). Fluroscence in-situ hybridization (FISH) was available in 2 patients who had del 13q14.3 with del 17p13.1 and t (4; 14) (FGFR3/IGH) . Out of 9 patients 8 were started on therapy and are being analyzed here.
Treatment intended was induction chemotherapy to achieve maximum response followed by tandem ASCT. Median number of chemotherapy cycles received was 4 (3-10 cycles). 3 patients proceeded to tandem ASCT. Most common toxicities during course of treatment were infections (gram negative bacterial and fungal) and neuropathy. Overall response rate (ORR) was 62.5% (5/8) with 4 patients achieving CR/VGPR and 1 PR. One patient had stable disease and 2 patients had progressive disease. After median follow up of 11.5 months (3-46 months), overall survival (OS) is 50% (4/8) and progression free survival (PFS) is 25% (2/8). OS and PFS of patient with or without ASCT is 67% (2/3) versus 40% (2/5) and 33% (1/3) versus 20% (1/5). Median PFS of entire cohort is 10 months (2-31 months) and 25 months (9-31 months) and 4 months (2-14 months) for patients with or without ASCT.
Conclusion: Primary PCLs are rare and aggressive in nature. Treatment with novel agents followed by high dose therapy and ASCT (tandem) might improve duration of remission and overall survival in some patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal